Vendor Spotlight
Latest Podcast
To listen to each episode and to earn your CE certificate, click into each episode below. Please note not all podcasts are CE approved.
Scope of Innovation: Single-Use Solutions for Surgical Success
January 16, 2026 Michael Vendor Spotlight 41:25 Comments Off on Scope of Innovation: Single-Use Solutions for Surgical Success
At the Tip of Safety: Sustainable Solutions for Instrument Protection
November 14, 2025 Michael Beyond Clean, Vendor Spotlight 38:23 Comments Off on At the Tip of Safety: Sustainable Solutions for Instrument Protection









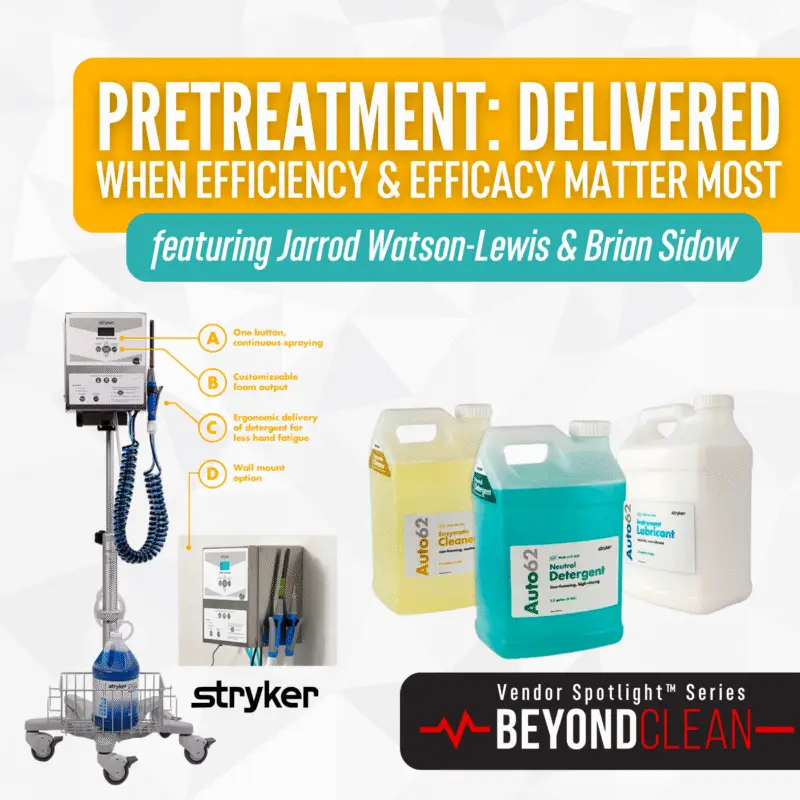
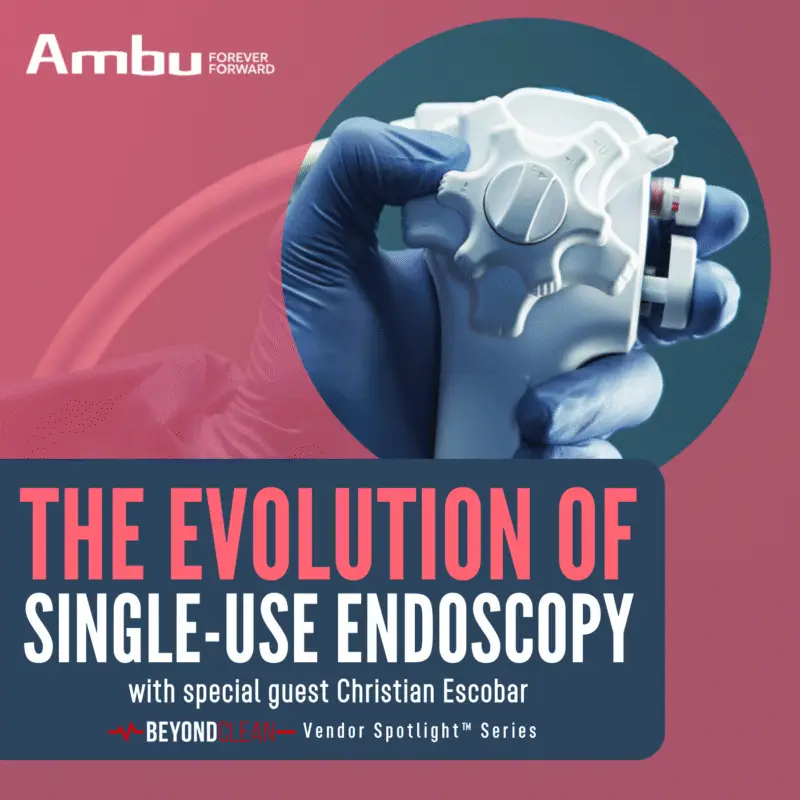






Scope of Innovation: Single-Use Solutions for Surgical Success
Perfect imaging every time. No repairs. No reprocessing. Could single-use arthroscopes be the answer? On this special Beyond Clean, First Case, Power Supply, and Transmission Control Vendor Spotlight™ co-release...